STELARA® Delivers Lasting Efficacy And Minimal Corticosteroid Use In UC
In The UNIFI-LTE, STELARA® Sustained Symptomatic Remission Through 2 Years[1]
Symptomatic Remission Over Time Through Week 92
For All Patients Who Were Randomised In The Maintenance Study (Nri Analysis)†[1]
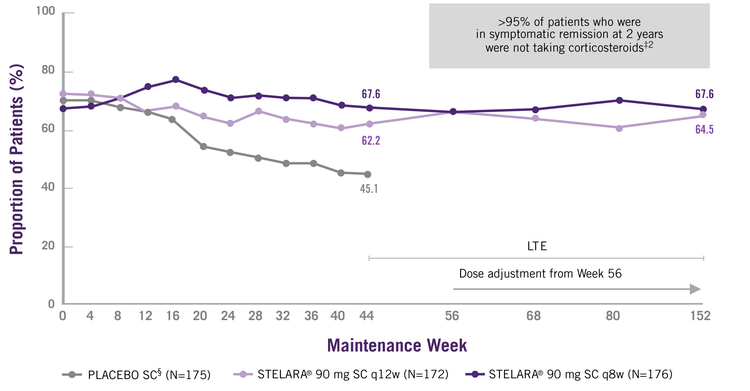
† Patients who were in clinical response 8 weeks after receiving a single STELARA® IV induction were randomised to placebo SC, STELARA® 90 mg SC q12w or STELARA® 90 mg SC q8w. All patients who completed Week 44 were eligible to enter and continue in the LTE at the investigator’s discretion. Symptomatic remission: stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0. Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit. Patients who had a prohibited change in UC medication, an ostomy or colectomy, or used a rescue medication after clinical flare or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC prior to the Week 44 visit were considered not to be in symptomatic remission.
‡ ITT population, dose-adjustment not considered a treatment failure.
§ Subsequent to completion of the maintenance study at Week 44, unblinding occurred and patients who were receiving placebo SC discontinued from the LTE.
Component not published? componentType is undefined.
Component not published? componentType is undefined.
In UC, STELARA® Allows For Minimal Corticosteroid Use For The Long Term¶††‡‡§§[1]
Non-responder Imputation Analysis Of Symptomatic Remission
And Corticosteroid-free Symptomatic Remission At Week 92[1]
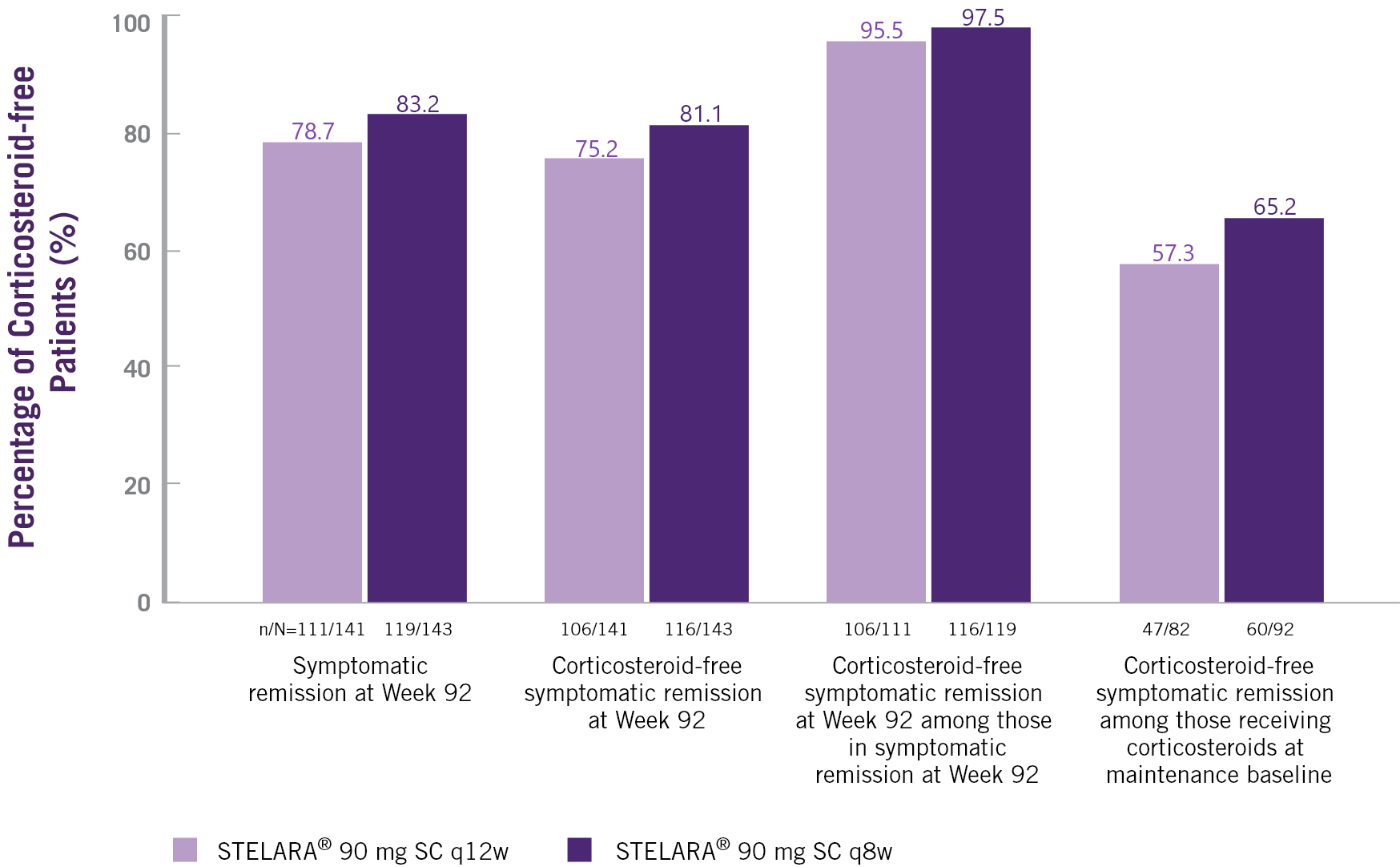
¶ Randomised group at maintenance Week 0 regardless of whether patients had a dose adjustment during the LTE.
†† Patients who had a missing value in corticosteroid use at a timepoint had their last available value carried forward to that timepoint.
‡‡ Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit.
§§ Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an adverse event of worsening of UC prior to the designated visit were considered not to be in symptomatic remission.
Component not published? componentType is undefined.
Component not published? componentType is undefined.
Histo-endoscopic Mucosal Healing
STELARA® is the first approved UC treatment to include in Phase III clinical trials (UNIFI induction and maintenance) the combined endpoint of histo-endoscopic mucosal healing[2]
In UC, Histologic Healing Is Associated With Better Clinical Outcomes:[3]
- Lower rates of disease relapse
- Reduced risk of cancer
- Reduction in need for surgery/hospitalisation
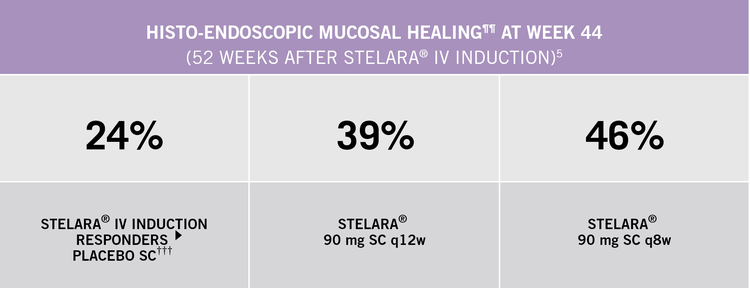
¶¶ Histo-endoscopic mucosal healing was defined as Mayo endoscopy score of 0 or 1 with neutrophil infiltration in <5% of crypts and on crypt destruction, erosion, ulcerations or granulation tissue.
††† Patients who were in clinical response with STELARA® IV induction dosing were randomised to placebo SC on entry into this maintenance study.
Component not published? componentType is undefined.
Component not published? componentType is undefined.
Component not published? componentType is undefined.
* Long-term remission is considered a target of ideal treatment in the clinical management of ulcerative colitis (UC).[4]
IV: Intravenous; NRI: Non-responder imputation analysis; UC: Ulcerative colitis; SC: Subcutaneous.
Prescribing Information
Adverse Events Reporting