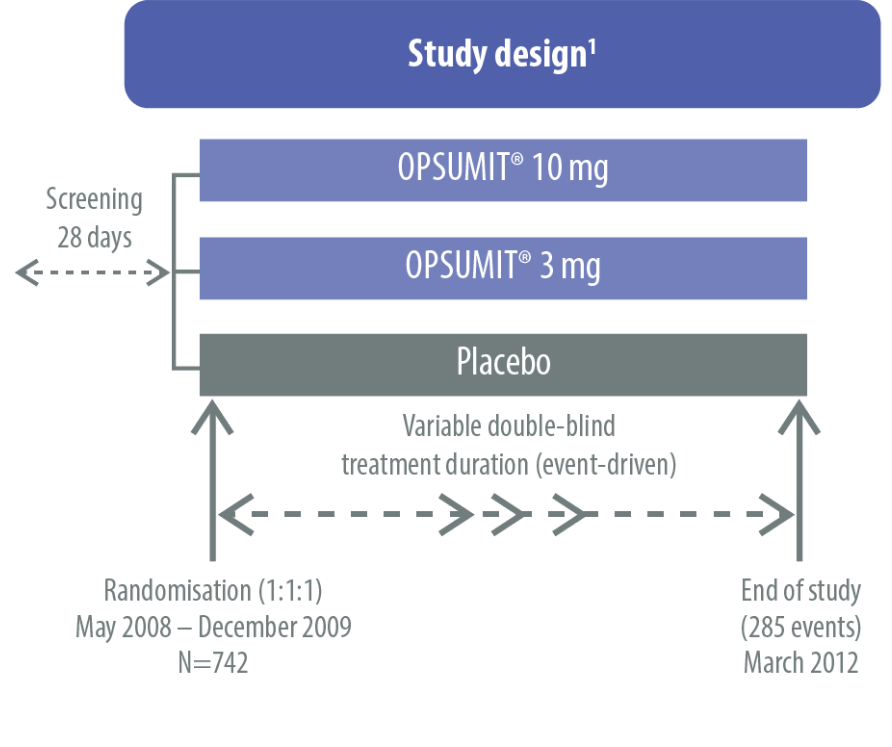
The safety and efficacy of OPSUMIT® were investigated in SERAPHIN, a Phase 3 study assessing the long-term benefits of OPSUMIT® in pulmonary arterial hypertension (PAH) patients. The primary endpoint in SERAPHIN was a composite endpoint of time to first morbidity or mortality event up to end of treatment.[1]
The SERAPHIN study was a multicentre, double-blind, randomised, placebo-controlled, parallel-group, event-driven, Phase 3 trial.[1]
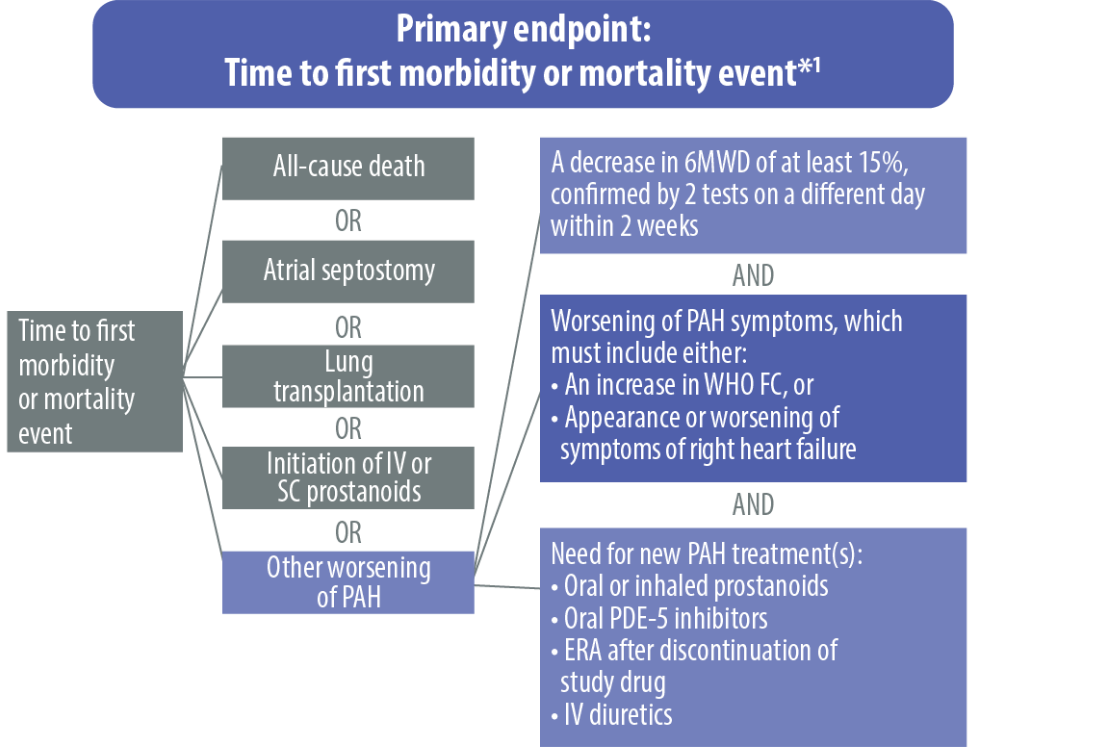
ERA, endothelin receptor antagonist; FC, functional class; IV, intravenous; PAH, pulmonary arterial hypertension; PDE-5, phosphodiesterase type 5; SC, subcutaneous; 6MWD, 6-minute walk distance; WHO, World Health Organization
*All events adjudicated by a blinded clinical events committee.
The SERAPHIN study assessed the safety and efficacy of OPSUMIT® across a broad range of patients with PAH.[1]
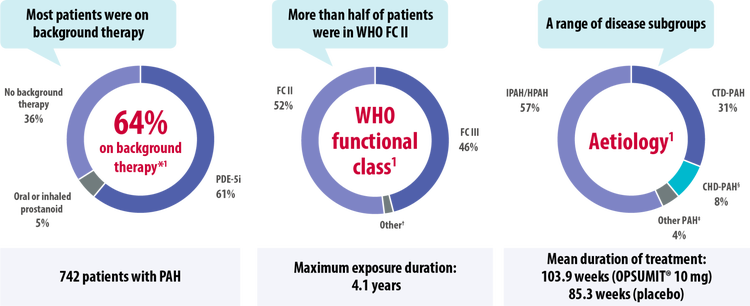
CHD, congenital heart disease; CTD, connective tissue disease; FC, functional class; HIV, human immunodeficiency virus; HPAH, heritable pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; PDE-5i, phosphodiesterase type-5 inhibitor; WHO, World Health Organization
*Patients may be on more than one background therapy.
†1 patient was in FC I, 14 patients in FC IV.
‡Other aetiologies included drug- or toxin-induced PAH (3%) and HIV-PAH (1%).
§Patients with repaired congenital systemic-to-pulmonary shunts.
The long-term efficacy of OPSUMIT® is based on SERAPHIN primary endpoint data showing a 45% reduction in the risk of morbidity-mortality* vs placebo. The treatment effect of OPSUMIT® was consistent across subgroups of aetiology, WHO FC and by monotherapy or in combination with another PAH therapy.[1][2][3][4][5]
OPSUMIT® Reduced The Risk Of A Morbidity Or Mortality* Event In Patients With PAH Vs Placebo[1]
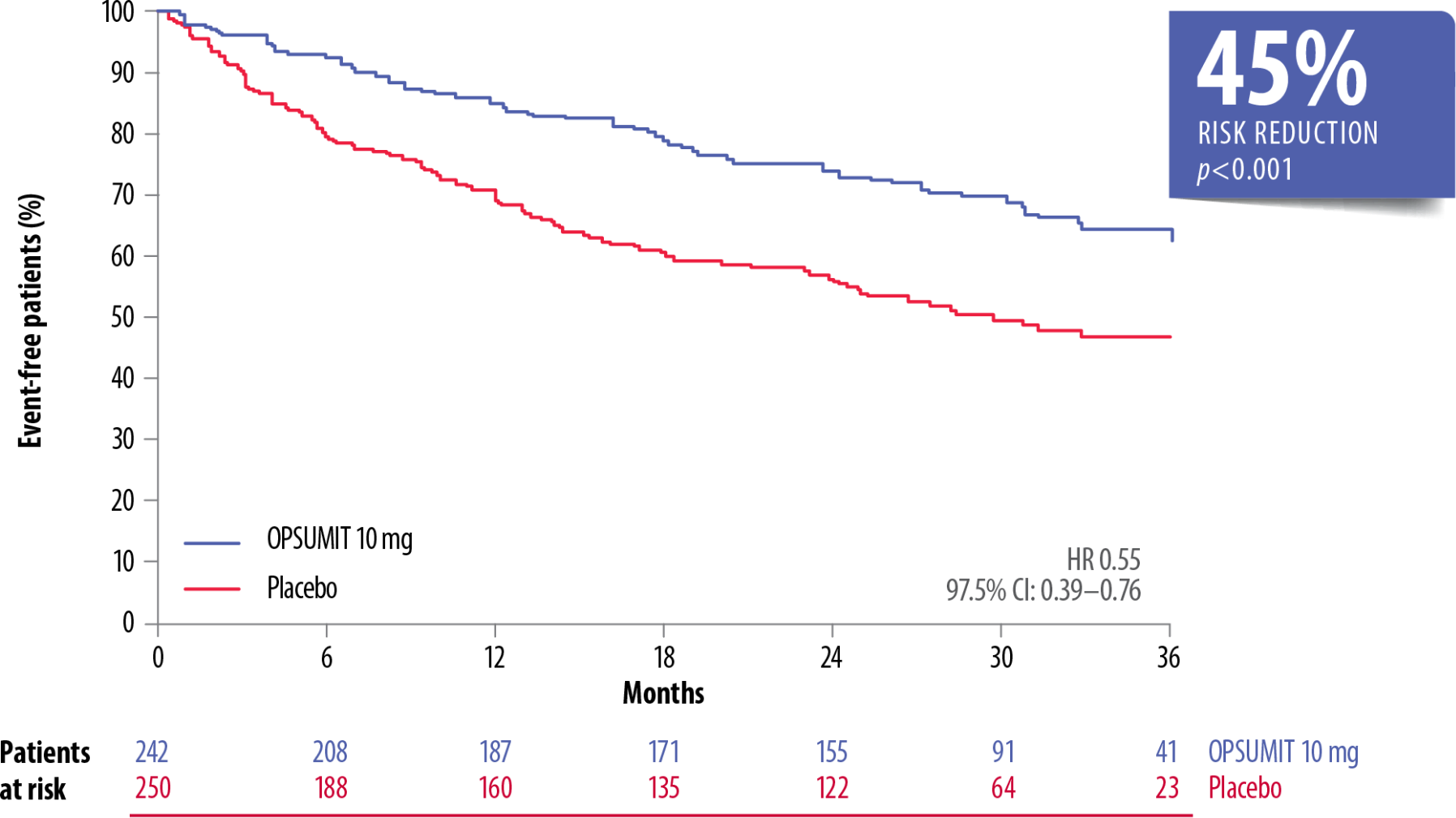
Adapted from Pulido et al. 2013[1]
CHD, congenital heart disease; CI, confidence interval; CTD, connective tissue disease; FC, functional class; HR, hazard ratio; PAH, pulmonary arterial hypertension; WHO, World Health Organization
*As measured by a composite primary morbidity-mortality endpoint. Results were driven by a decrease in PAH worsening and do not apply to mortality on its own.
Consistent with the primary endpoint in the overall population, OPSUMIT® reduced the risk of a morbidity-mortality event* vs placebo in patients with CTD-PAH by 42% (HR 0.58; 95% CI: 0.33–1.02)[2][3] and in patients with CHD-PAH† by 59% (HR 0.41; 95% CI: 0.13–1.31).[2]
*As measured by a composite primary morbidity-mortality endpoint. Results were driven by a decrease in disease progression and hospitalisations due to PAH; they do not apply to mortality on its own.
†Patients with repaired congenital systemic-to-pulmonary shunts.
OPSUMIT® reduced the risk of a morbidity or mortality event* vs placebo by 38% in patients on monotherapy with a PDE-5i or oral/inhaled prostanoid.[4]
OPSUMIT® Reduced The Risk Of A Morbidity Or Mortality* Event In Combination Therapy Vs Placebo[4]
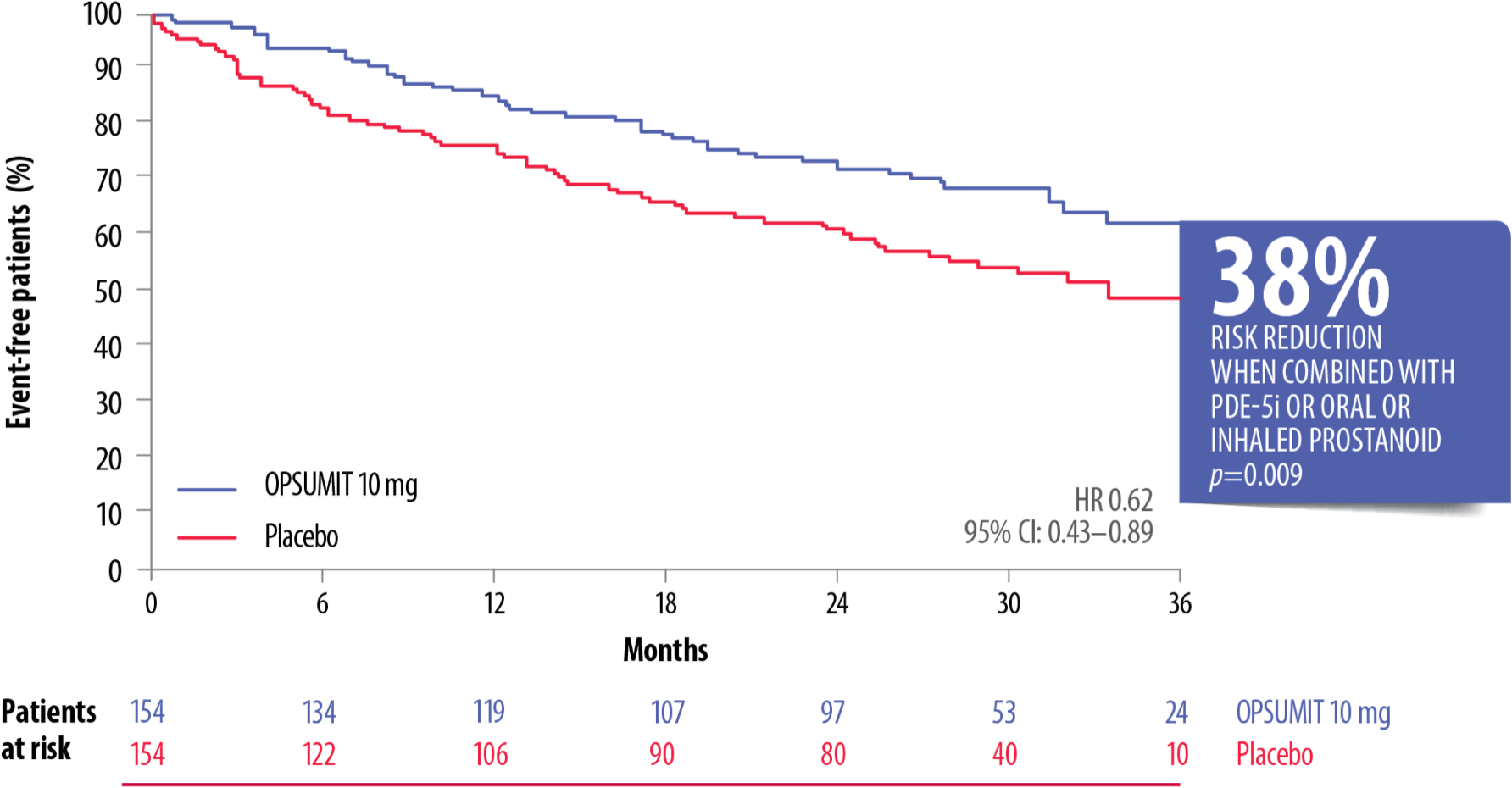
Adapted from Pulido et al. 2013[4]
CI, confidence interval; HR, hazard ratio; PAH, pulmonary arterial hypertension; PDE-5i, phosphodiesterase type-5 inhibitor
*As measured by a composite primary morbidity-mortality endpoint. Results were driven by a decrease in PAH worsening and do not apply to mortality on its own.
OPSUMIT® showed a consistent beneficial treatment effect on long-term outcomes in PAH patients across WHO FC subgroups.[5]
OPSUMIT® Reduced The Risk Of A Morbidity-Mortality Event* Vs Placebo In WHO FC I/II And FC III/IV Patients[5]
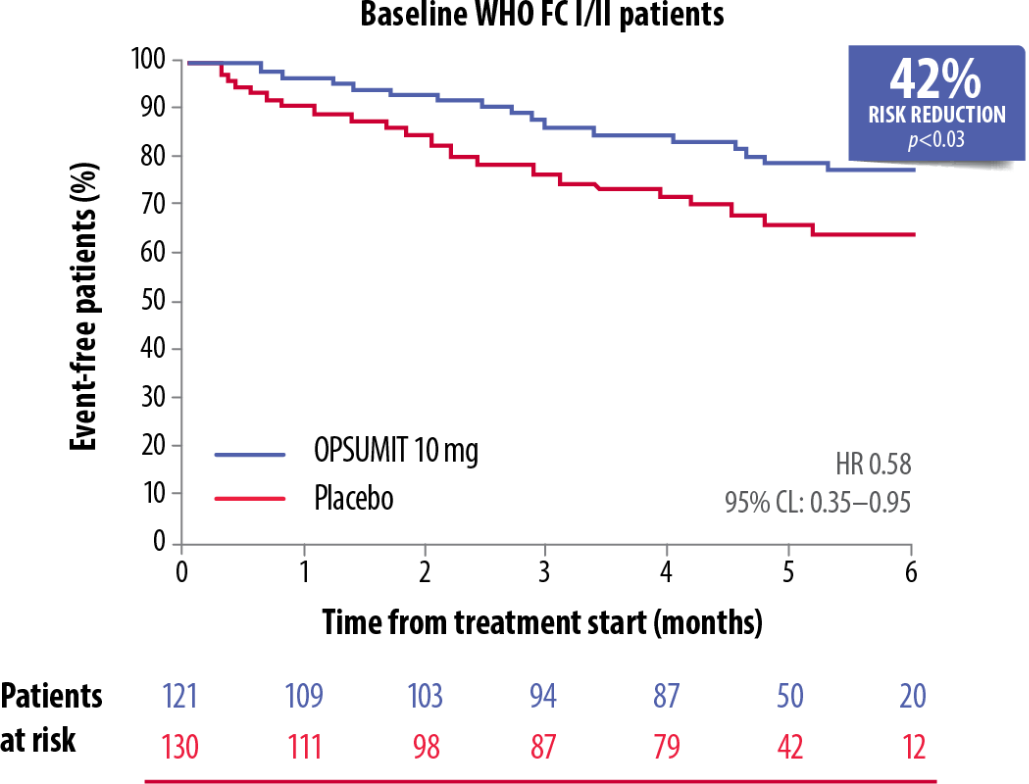
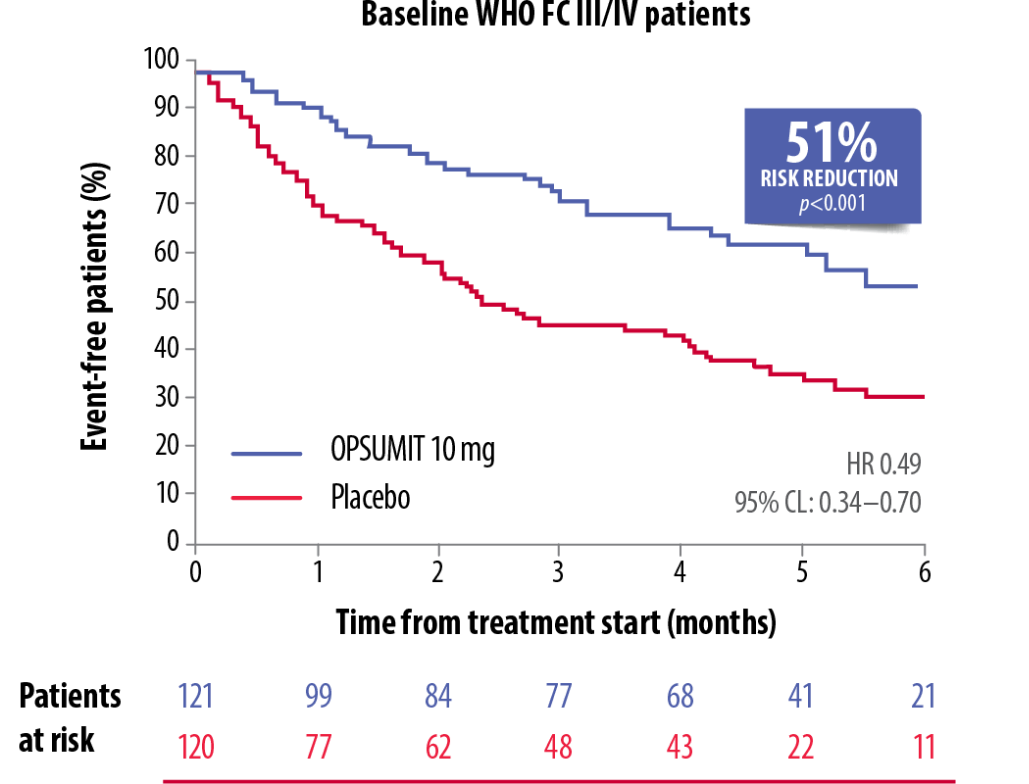
Adapted from Channick et al. 2014[5]
CL, confidence limit; FC, functional class; HR, hazard ratio; PAH, pulmonary arterial hypertension; WHO, World Health Organization
*As measured by a composite primary morbidity-mortality endpoint. Results were driven by a decrease in PAH worsening and do not apply to mortality on its own.
OPSUMIT® reduced the risk of a morbidity-mortality event* vs placebo in both incident and prevalent PAH patients.[6]
OPSUMIT® Reduced The Risk Of Morbidity-Mortality* Vs Placebo In Treatment-Naïve Incident PAH† Patients[6]
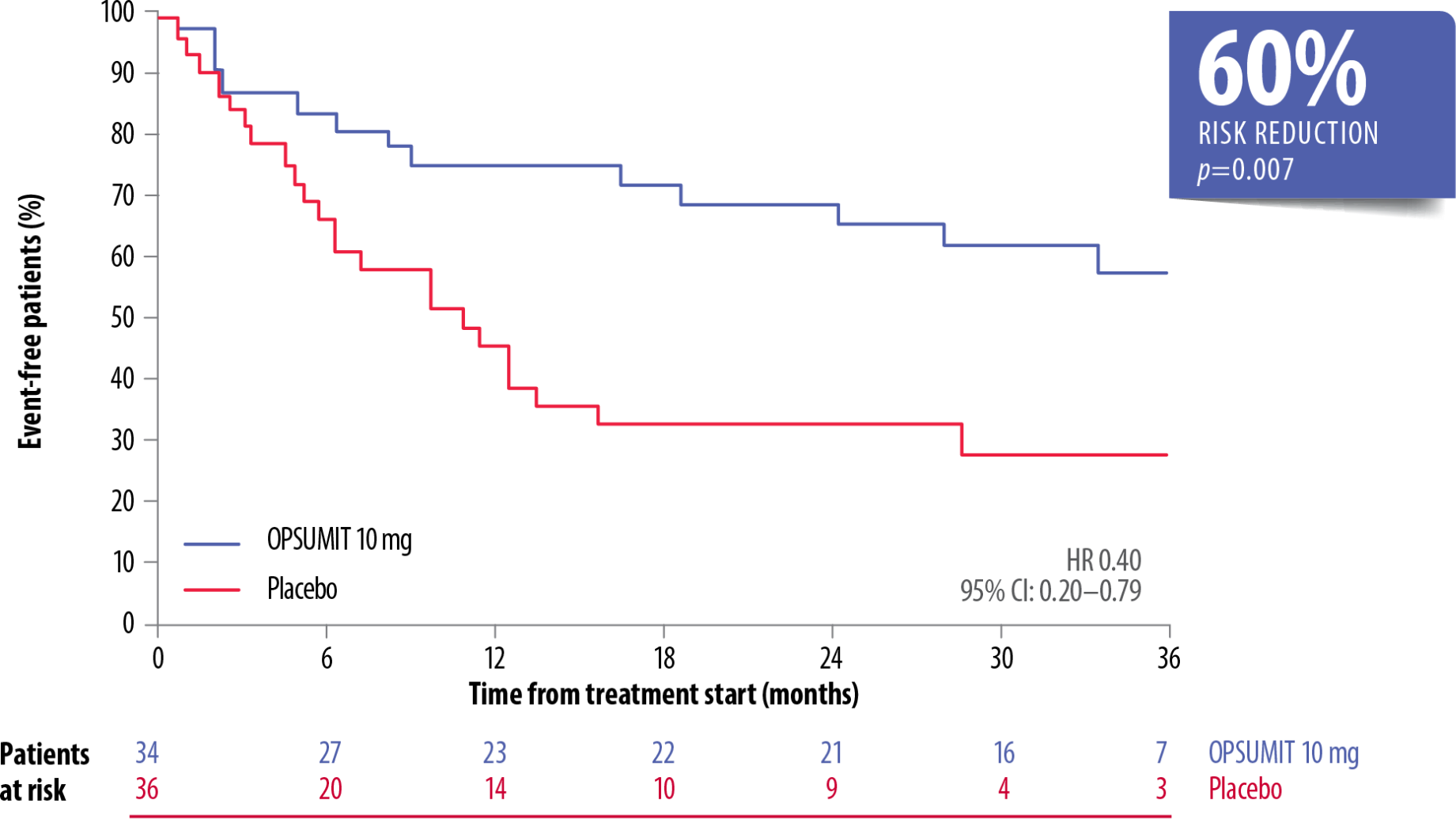
Adapted from Simonneau et al. 2015[6]
CI, confidence interval; HR, hazard ratio; PAH, pulmonary arterial hypertension
*As measured by a composite primary morbidity-mortality endpoint. Results were driven by a decrease in PAH worsening and do not apply to mortality on its own.
†Patients were classified by time from diagnosis to enrolment as incident (≤6 months; n=110) or prevalent (>6 months; n=157).
OPSUMIT® reduced the risk of morbidity-mortality* vs placebo by 57% in incident patients on monotherapy or combination therapy (HR 0.43, 95% CI: 0.25–0.73; p=0.001).[6]
OPSUMIT® Reduced The Risk Of Morbidity-Mortality* Vs Placebo In Treatment-Naïve Prevalent PAH† Patients[6]
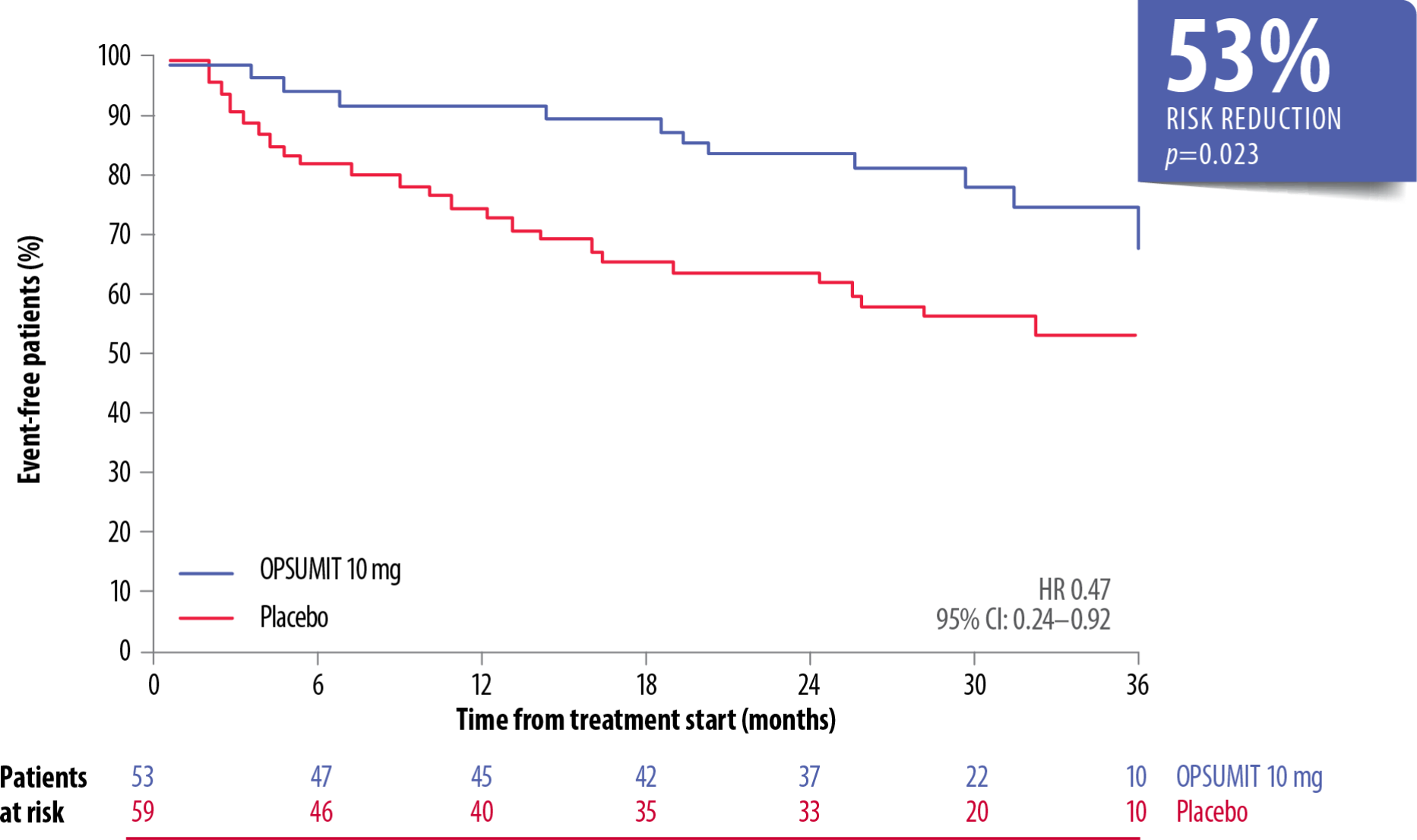
Adapted from Simonneau et al. 2015[6]
CI, confidence interval; HR, hazard ratio; PAH, pulmonary arterial hypertension
*As measured by a composite primary morbidity-mortality endpoint. Results were driven by a decrease in PAH worsening and do not apply to mortality on its own.
†Patients were classified by time from diagnosis to enrolment as incident (≤6 months; n=110) or prevalent (>6 months; n=157).
OPSUMIT® reduced the risk of morbidity-mortality* vs placebo by 41% in prevalent patients on monotherapy or combination therapy (HR 0.59, 95% CI: 0.42–0.84; p=0.003).[6] There was no heterogeneity of the treatment effect with OPSUMIT® across the incident and prevalent PAH patient cohorts (p=0.602).[6]
OPSUMIT® is the only ERA to demonstrate long-term survival at 7 years, with over 60% of patients still alive in the open-label extension of the SERAPHIN study.*[7]
Long-Term Survival Demonstrated Up To 7 Years With OPSUMIT® In The SERAPHIN Open-Label Extension[7]
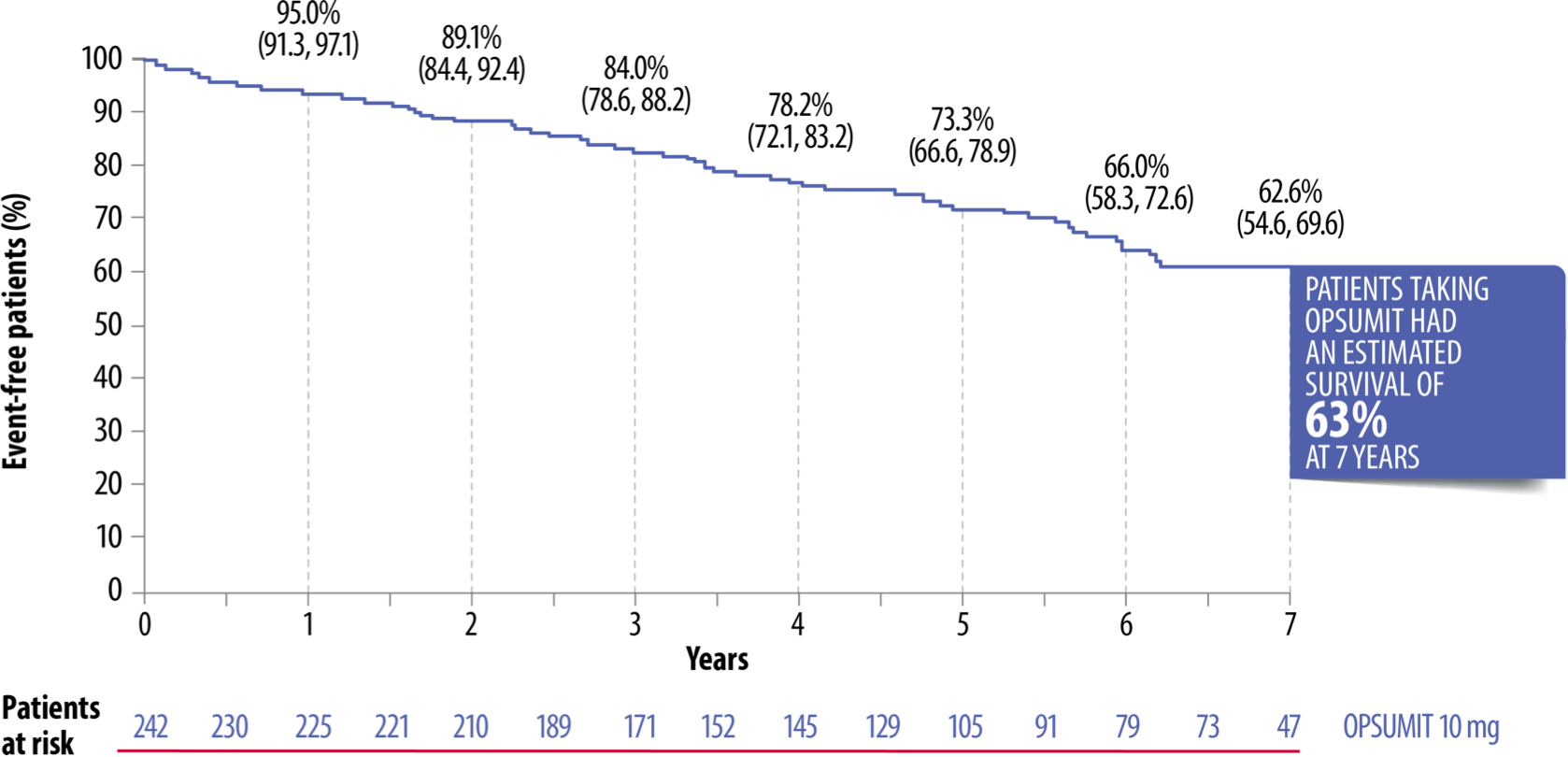
Adapted from Souza et al. 2017[7]
CHD, congenital heart disease; CTD, connective tissue disease; ERA, endothelin receptor antagonist; FC, functional class; HIV, human immunodeficiency virus; HPAH, hereditary pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; PDE-5i, phosphodiesterase type-5 inhibitor
*182 patients randomised to OPSUMIT® in the double-blind study continued on OPSUMIT® in the open-label extension. This included mainly FC II–III patients with IPAH/HPAH or PAH associated with CTD, CHD, HIV or drugs/toxins; 64% of patients were on background therapy at baseline with 62% on a PDE-5i and 6% on an oral or inhaled prostanoid. Survival was estimated by the Kaplan-Meier method.
Real-world data from the OPUS and OrPHeUS registries demonstrated comparable survival in CTD-PAH and IPAH/HPAH patients treated with OPSUMIT®.*[8][9]
Similar 2-Year Survival Estimates In CTD-PAH Patients Vs IPAH/HPAH Patients[9]
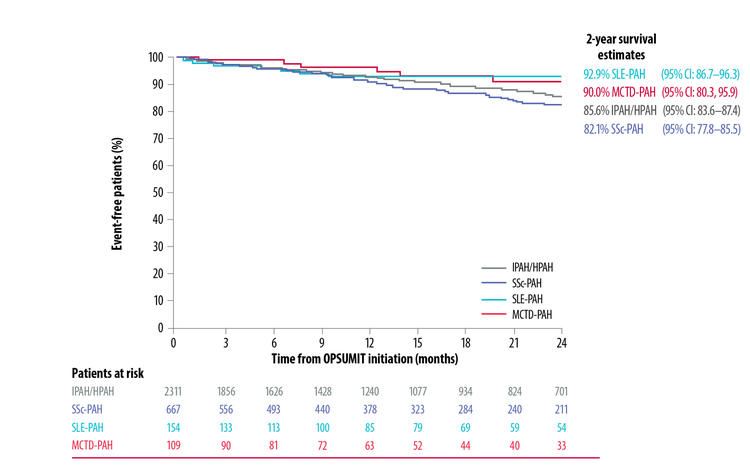
Adapted from McLaughlin et al. 2019[9]
CTD, connective tissue disease; HPAH, heritable pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; MCTD, mixed connective tissue disease; PAH, pulmonary arterial hypertension; SLE, systemic lupus erythematosus; SSc, systemic sclerosis
*Patient follow-up was according to routine clinical practice rather than scheduled visits, and comparison between the populations was descriptive.
Learn More About OPSUMIT®
Advice on how to manage your OPSUMIT® patients, including details on dosage and side effects.
Details from the 2015 ESC/ERS guidelines to help you achieve and/or maintain a low-risk status for your patients with PAH.