
Patient Management
- The recommended dosage of OPSUMIT® is 10 mg once daily for oral administration
- No titration is necessary
- Tablets can be administered with or without food
- Tablets should not be split, crushed or chewed
OPSUMIT® has a well-established safety profile.[1][2] The majority of adverse events in the SERAPHIN study were mild to moderate.[2]
Most Frequent Adverse Events In The SERAPHIN Study[2]
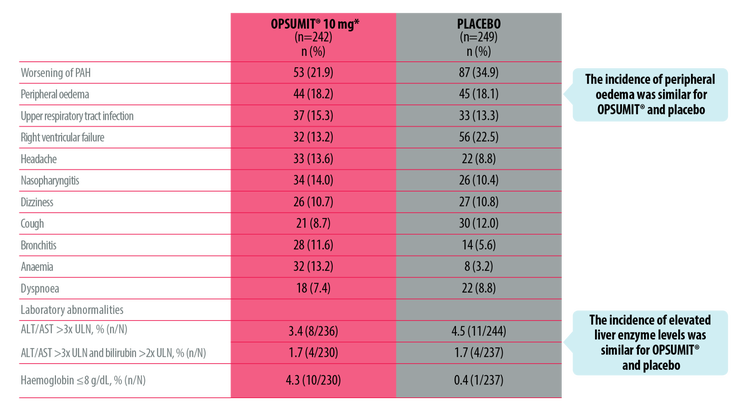
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LFT, liver function test; PAH, pulmonary arterial hypertension; PDE-5i, phosphodiesterase type-5 inhibitor; ULN, upper limit of normal
*At baseline, 61.4% of SERAPHIN patients were receiving a PDE-5i and 5.4% were receiving oral or inhaled prostanoid as background therapy.
The overall incidence of treatment discontinuations due to adverse events with OPSUMIT® was similar to placebo (10.7% and 12.4%, respectively).[2]
For complete information about the safety and tolerability of OPSUMIT®, please consult the local Summary of Product Characteristics.
Learn More About OPSUMIT®
Details of the SERAPHIN study, where the safety and efficacy of OPSUMIT® were investigated in a Phase 3 study to assess the long-term benefits for pulmonary arterial hypertension (PAH) patients.
Details from the 2015 ESC/ERS guidelines to help you achieve and/or maintain a low-risk status for your patients with PAH.