The safety and tolerability of UPTRAVI® were investigated in the GRIPHON trial, the largest outcomes trial conducted in pulmonary arterial hypertension (PAH) to date.[1][2] The majority of adverse events (AEs) in the trial were mild to moderate and manageable with treatment. Additionally, prostacyclin pathway-associated AEs were less frequent after the individualised maintenance dose was reached.[1][3]
Prostacyclin-associated AEs
Most common AEs are typical of drugs that activate the prostacyclin pathway.[1][3]
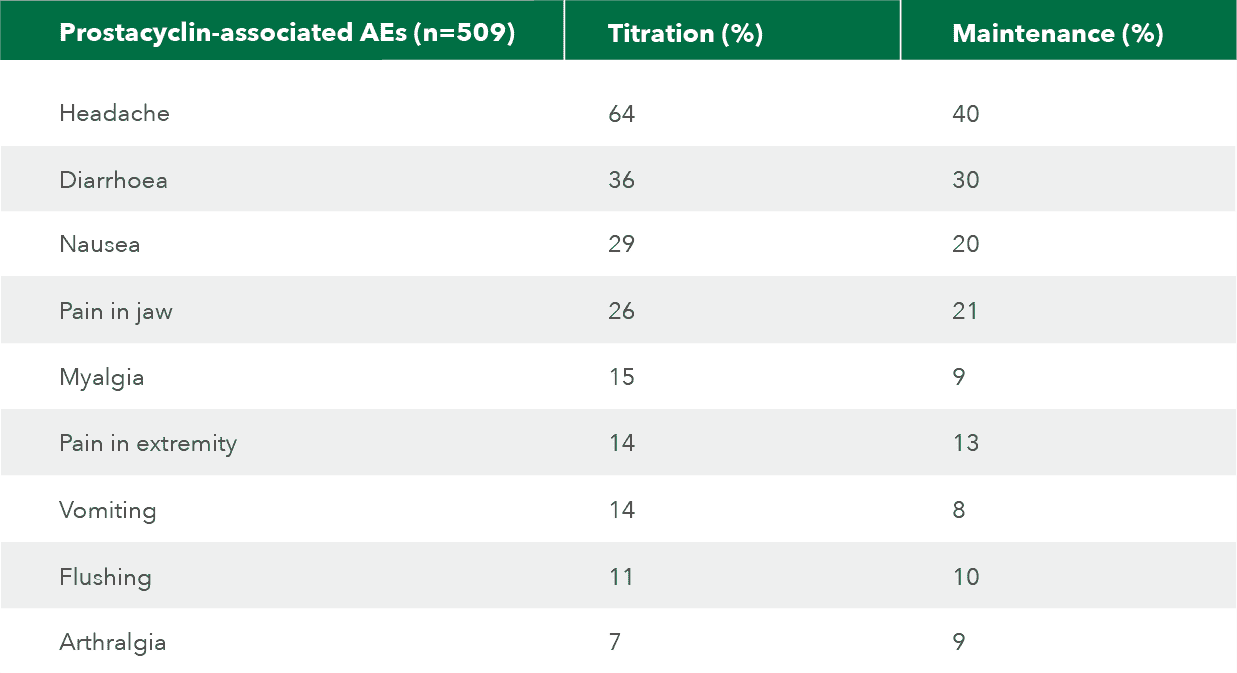
Adapted from Uptravi Israel PI, 7/2022[3]
Adverse events
Overall, 14% of patients in the UPTRAVI® group and 7% of patients in the placebo group prematurely discontinued treatment due to an AE in GRIPHON.[1] The majority of AEs were mild to moderate and manageable with treatment.[1][3]
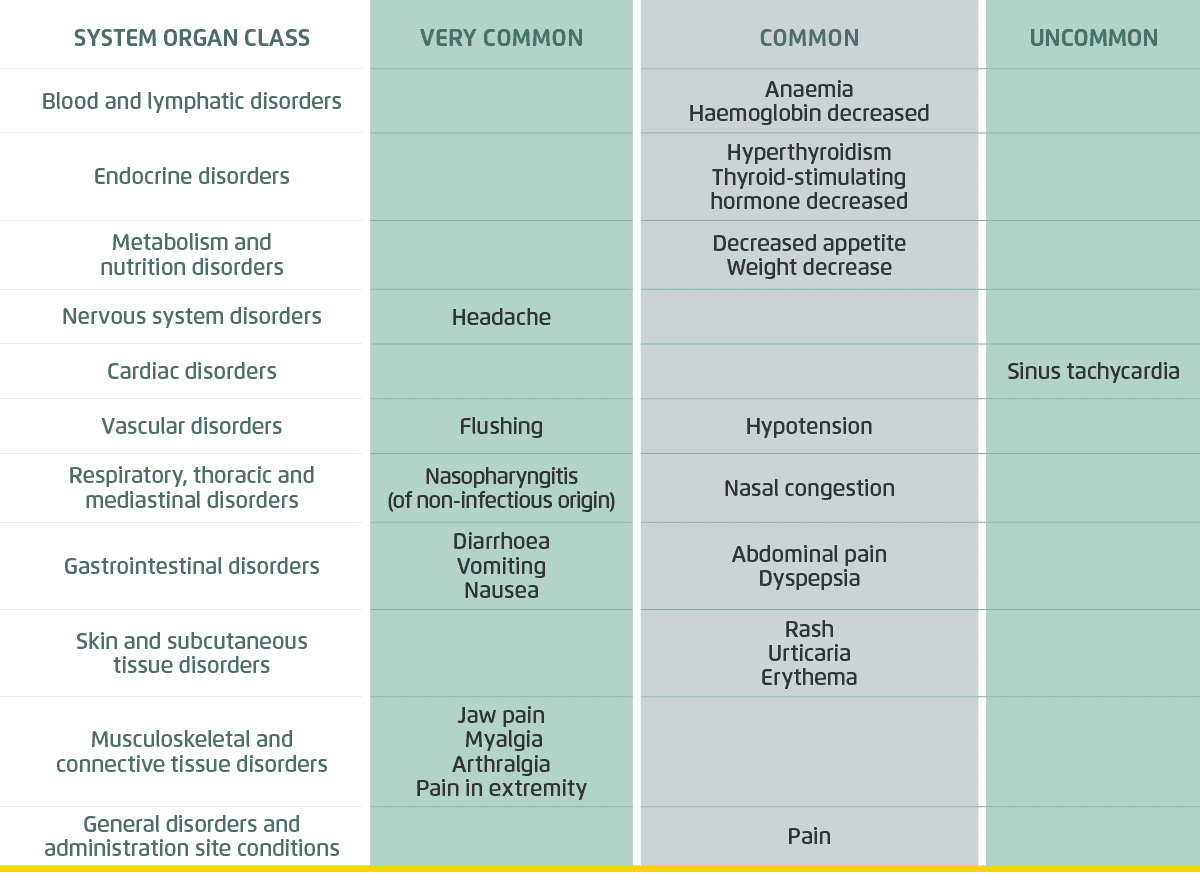
Adapted from Uptravi Israel PI.[3]
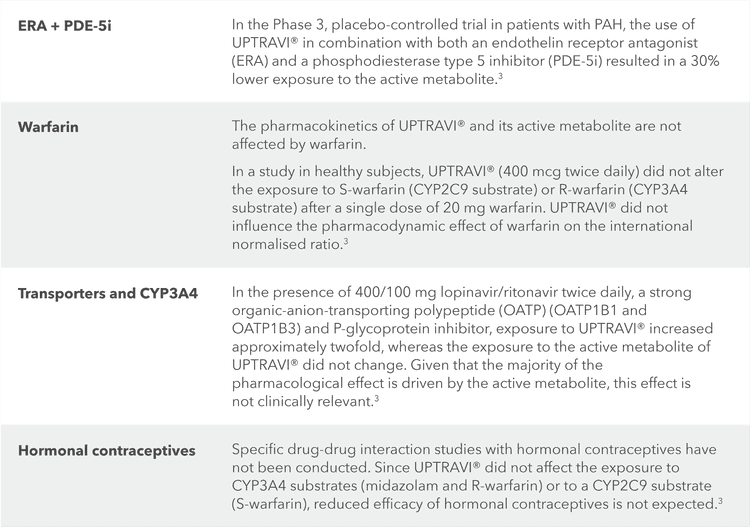
Drug-drug interactions
For further information regarding Opsumit® (macitentan) or Uptravi® (Selexipag) including full indications, all adverse effects and data please refer to the Israeli MOH prescribing information: https://israeldrugs.health.gov.il/#!/byDrug