The efficacy and safety of UPTRAVI® were investigated in the GRIPHON trial, the largest outcomes trial conducted in pulmonary arterial hypertension (PAH) to date.[1][2]
Study design and composite primary endpoint
The GRIPHON trial was a multicentre, double-blind, randomised, parallel-group, placebo-controlled, event-driven Phase 3 trial that assessed the safety and efficacy of UPTRAVI® across a broad range of patients with PAH. UPTRAVI® efficacy was demonstrated using a composite long-term outcomes primary endpoint that included PAH-related hospitalisation, disease progression events and death.[1]
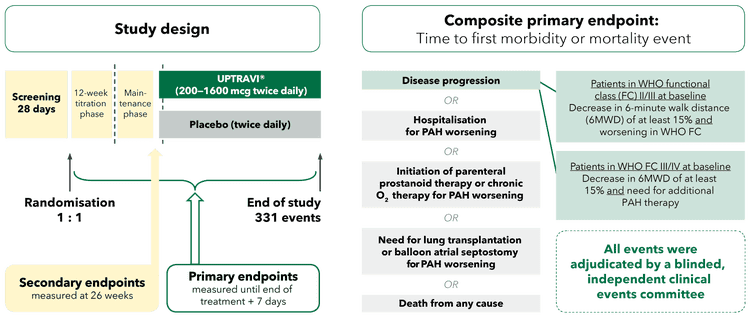
Adapted from Sitbon et al. 2015[1]
Patient population
The GRIPHON trial assessed the efficacy and safety of UPTRAVI® across a broad range of patients with PAH.[1]
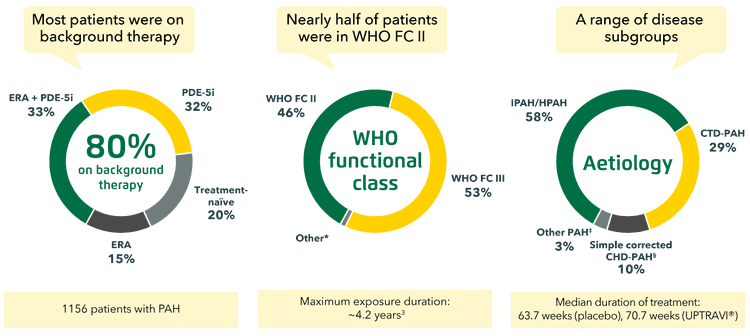
Adapted from Sitbon et al. 2015[1] and Uptravi® PI, 7/ 2022[3]
*9 patients were in WHO FC I, 11 patients in WHO FC IV.[1]
‡Other aetiologies included drug- or toxin-induced PAH (2%) and HIV-PAH (1%).[1]
§Patients with repaired congenital systemic-to-pulmonary shunts.[1]
Long-term efficacy with UPTRAVI®
Evidence for the long-term efficacy of UPTRAVI® is based on GRIPHON primary endpoint data, showing a significant reduction in the risk of disease progression vs placebo in patients with PAH.[1]
In the GRIPHON trial, UPTRAVI® significantly reduced the risk of disease progression by 40% vs placebo.[1]
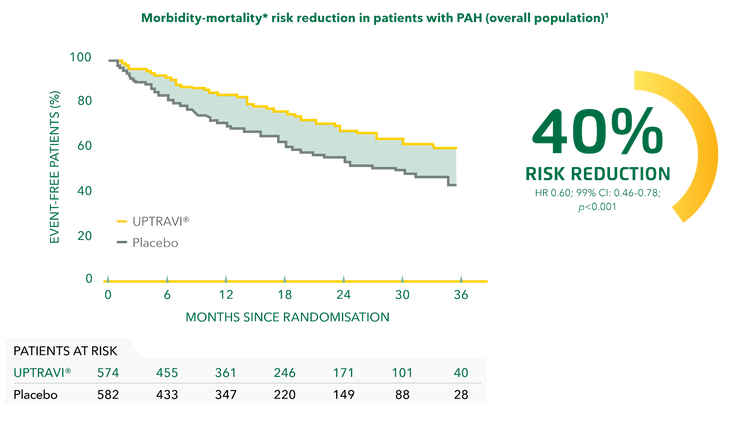
Adapted from Sitbon et al. 2015[1]
*Composite primary endpoint; results do not apply to mortality on its own.[1]
UPTRAVI® is the only oral prostacyclin pathway agent proven to reduce PAH-related hospitalisation risk vs placebo.*[3]
*Component of the composite morbidity-mortality primary endpoint.[3]
UPTRAVI®: No other prostacyclin pathway agent has 7-year survival data*[3]
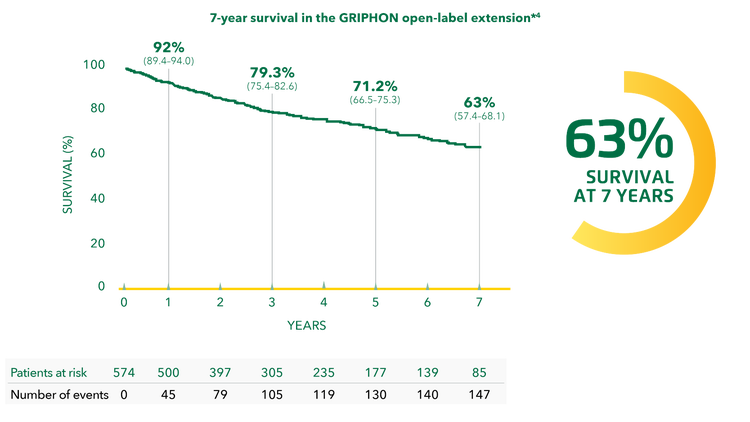
Adapted from Galiè et al. 2022[4]
*Given that additional PAH treatment was initiated in a small proportion of patients and that there was no control group in the extension study, the survival benefit of UPTRAVI® cannot be confirmed from these data.[4]
Efficacy in a broad range of patients with PAH
PAH is a rare disease, with an estimated prevalence of 10–52 cases per million.[5] In certain at-risk groups, the prevalence can be substantially higher.[6][7]
Patients with PAH associated with connective tissue disease (CTD)
Consistent with the primary endpoint outcomes of the overall population, UPTRAVI® reduced the risk of disease progression vs placebo in patients with CTD-PAH by 41% (HR 0.59; 95% CI: 0.41–0.85).[8]
The treatment effect with UPTRAVI® was consistent in patients with CTD-PAH across CTD subtypes, including systemic sclerosis (SSc)-associated and systemic lupus erythematosus (SLE)-associated PAH (see below). UPTRAVI® also reduced the risk of disease progression vs placebo by 32% in patients with mixed CTD-PAH/other CTD-PAH (HR 0.68; 95% CI: 0.31–1.47).[8]
UPTRAVI® reduced the risk of disease progression by 44% vs placebo in patients with SSc-PAH.[8]
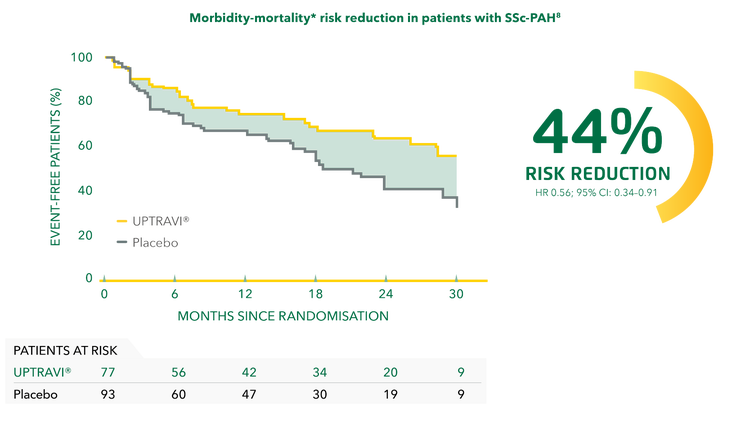
Adapted from Gaine et al. 2017[8]
*Composite primary endpoint; results do not apply to mortality on its own.[8]
UPTRAVI® reduced the risk of disease progression by 34% vs placebo in patients with SLE-PAH.[8]
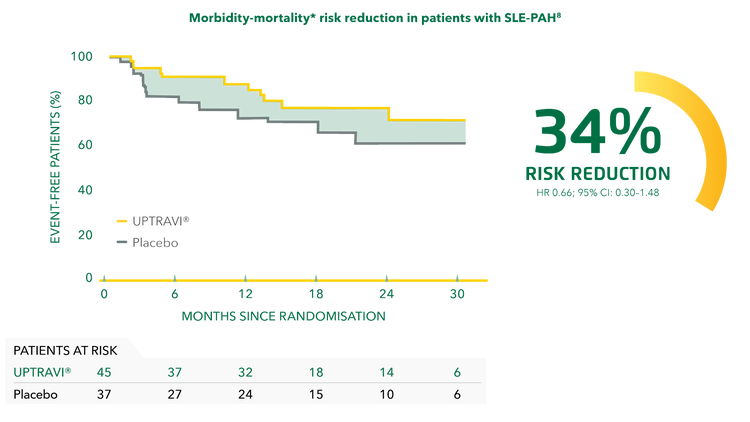
Adapted from Gaine et al. 2017[8]
*Composite primary endpoint; results do not apply to mortality on its own.[8]
Patients with PAH associated with simple corrected congenital heart disease (CHD)
Consistent with the primary endpoint in the overall population, UPTRAVI® reduced the risk of disease progression vs placebo in patients with simple corrected CHD-PAH* by 42%.[9]
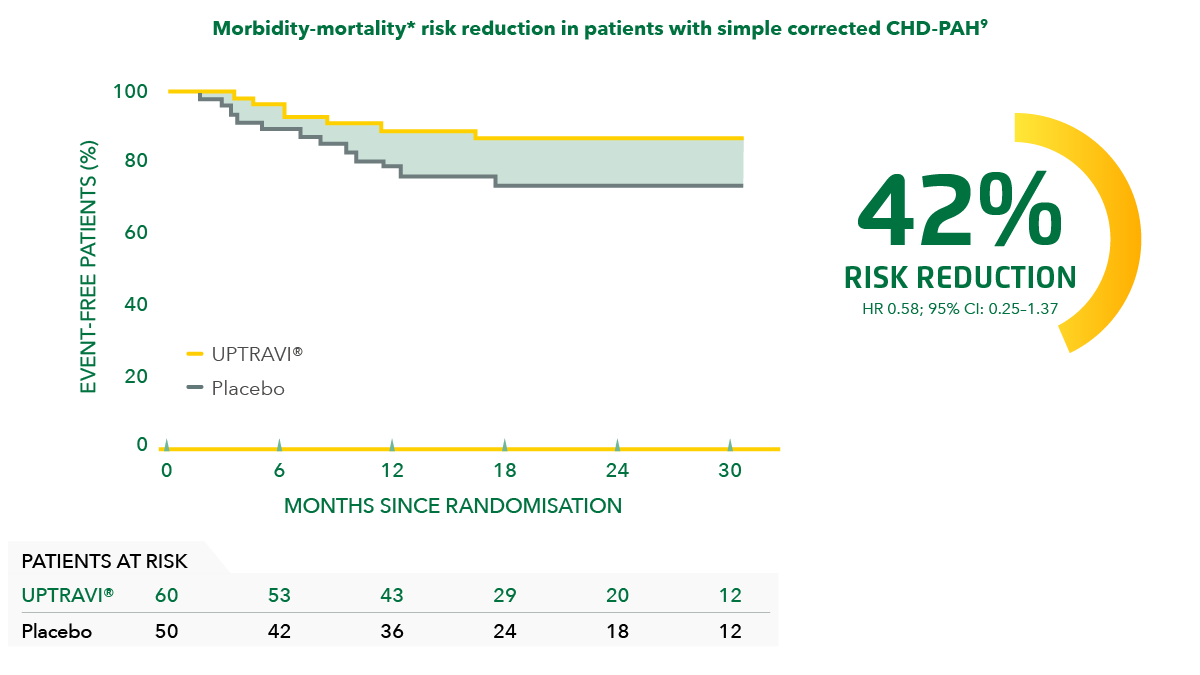
Adapted from Beghetti et al. 2019[9]
*Patients with repaired congenital systemic-to-pulmonary shunts.[9]
‡Composite primary endpoint; results do not apply to mortality on its own.[9]
Patients with cardiovascular (CV) comorbidities
UPTRAVI® reduced the risk of disease progression in patients with PAH and <3 CV comorbidities* by 34% vs placebo. In patients with PAH with ≥3 CV comorbidities, UPTRAVI® reduced the risk of disease progression by 50% vs placebo.[10]
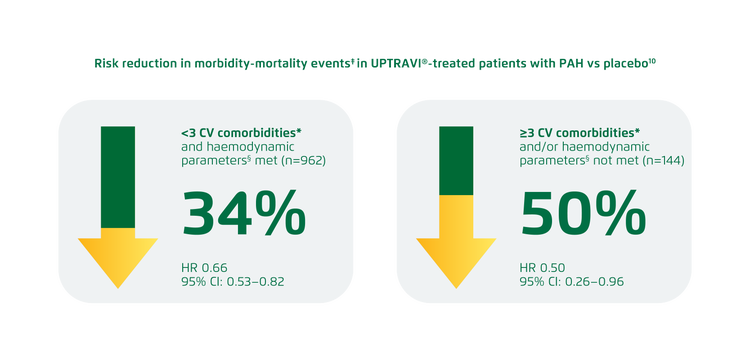
Adapted from Rosenkranz et al. 2022[10]
The treatment effect of UPTRAVI® is not influenced by the number of comorbidities or the presence of a specific comorbidity (interaction p value=0.432).[10]
UPTRAVI® is contraindicated in patients with certain CV comorbidities. Please refer to the full Uptravi® PI. [11]
*CV comorbidities were defined as: BMI >30 kg/m2, history of essential hypertension, diabetes mellitus or historical evidence of significant coronary artery disease.[10]
‡Composite endpoint of morbidity-mortality. Results do not apply to mortality on its own.[10]
§Haemodynamic parameter cut-offs required a PAWP/LVEDP of ≤12 mmHg when PVR was ≥3.75 to <6.25 WU. If PVR was ≥6.25 WU, the PAWP/LVEDP had to be ≤15 mmHg.[10]
Patients with different numbers of low-risk criteria
UPTRAVI® reduced the risk of disease progression by 41%, 35% and 42% in the subgroup of patients with 0, 1 and 2 low-risk criteria, respectively, compared with placebo.*[12]
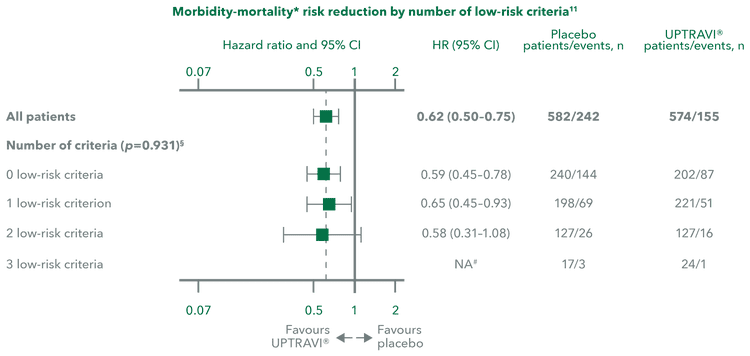
Adapted from Sitbon et al. 2020[12]
*Post hoc analysis assessing the effect of UPTRAVI® on risk profile from baseline to Week 26 compared with placebo in the GRIPHON population using both the non-invasive French registry and REVEAL 2.0 risk assessment methods.[12]
‡Composite primary endpoint; results do not apply to mortality on its own.[12]
§p value for interaction.[12]
#HRs could not be calculated in patients with 3 low-risk criteria because of the low numbers of morbidity/mortality events that occurred.[12]
Combination therapy
In the GRIPHON trial, the effect of UPTRAVI® was consistent with the primary endpoint for all background therapy subgroups. UPTRAVI® reduced the risk of disease progression vs placebo by 43% in patients not receiving background therapy at baseline, by 42% in patients receiving phosphodiesterase type 5 inhibitor (PDE-5i) monotherapy at baseline and by 34% in patients receiving endothelin receptor antagonist (ERA) monotherapy at baseline.[13]
UPTRAVI® also improved long-term outcomes for patients when added in triple combination therapy, including those in World Health Organization (WHO) functional class (FC) II and III (see below).[14]
Adding UPTRAVI® to double combination therapy reduced the risk of disease progression by 37% vs placebo in the overall population.[14]
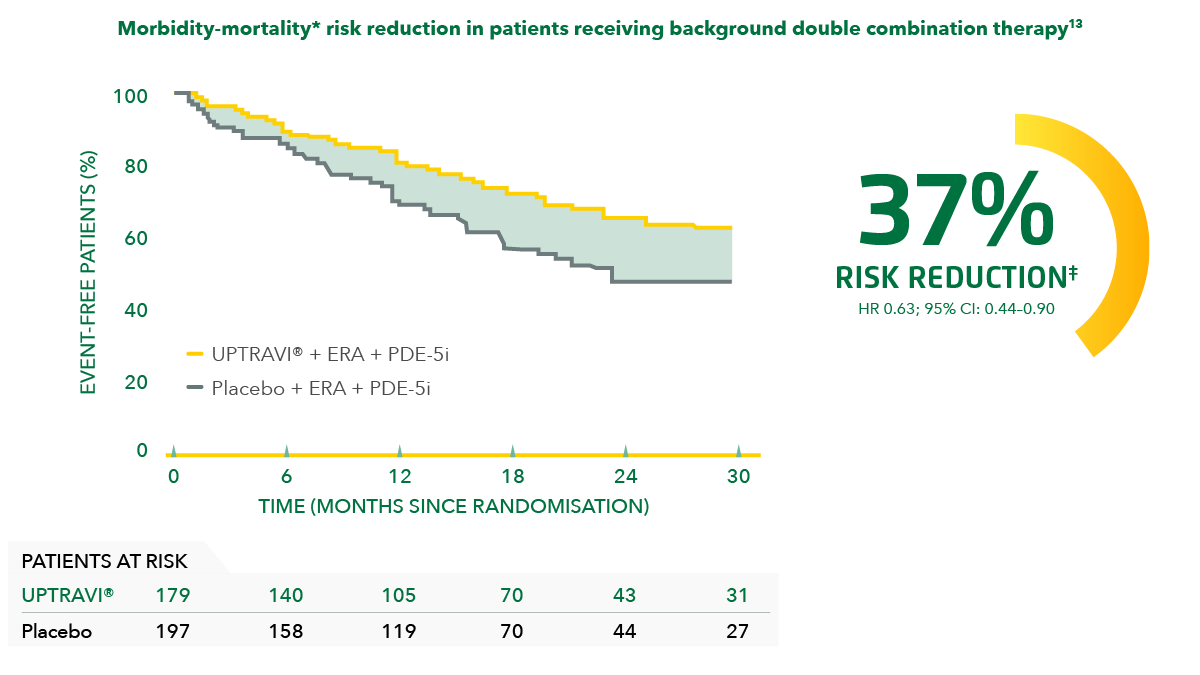
Adapted from Coghlan et al. 2018[14]
*Composite primary endpoint; results do not apply to mortality on its own.[14]
‡Post hoc analysis of patients in GRIPHON on background therapy with an ERA and a PDE-5i at baseline.[14]
Adding UPTRAVI® to double combination therapy reduced the risk of disease progression by 64% vs placebo in WHO FC II patients.[14]
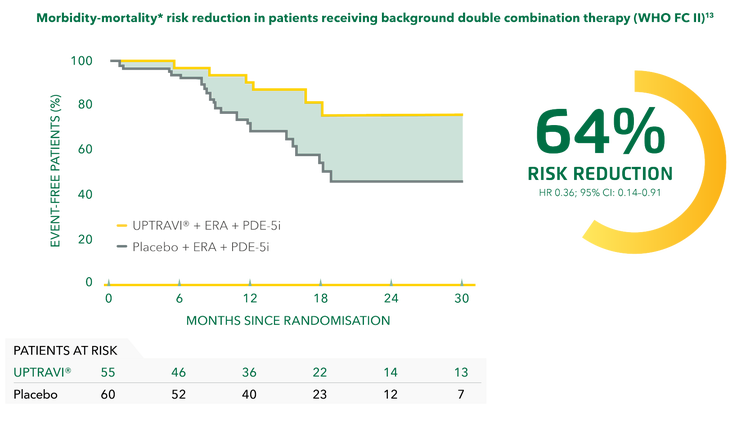
Adapted from Coghlan et al. 2018[14]
*Composite primary endpoint; results do not apply to mortality on its own.[14]
‡Post hoc analysis of patients in GRIPHON on background therapy with an ERA and a PDE-5i at baseline.[14]
Adding UPTRAVI® to double combination therapy reduced the risk of disease progression by 26% vs placebo in WHO FC III patients.[14]
Adapted from Coghlan et al. 2018[14]
*Composite primary endpoint; results do not apply to mortality on its own.[14]
‡Post hoc analysis of patients in GRIPHON on background therapy with an ERA and a PDE-5i at baseline.[14]
Plan from the start to add UPTRAVI® within 6 months of diagnosis if the patient is not fully at low risk on dual therapy[15][16][17]
Early* initiation of UPTRAVI® improved long-term outcomes vs placebo to a greater degree than when initiated later.* Treatment with UPTRAVI® reduced the risk of disease progression vs placebo when initiated within 6 months of diagnosis and when initiated after 6 months from diagnosis.[15]
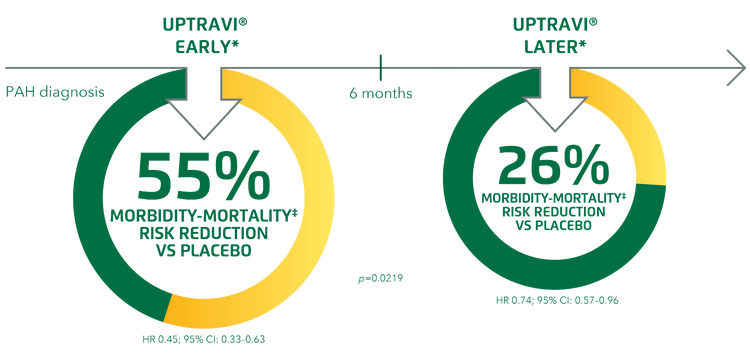
Adapted from Gaine et al. 2021[15]
At baseline, 53.7% of patients who were diagnosed early were found to be in WHO FC I/II, while 42.7% of patients diagnosed later were in WHO FC I/II.[15]
*Post hoc analysis in time-from-diagnosis subgroups in GRIPHON. Early: ≤6 months from diagnosis, later: >6 months from diagnosis.[15]
‡Composite primary endpoint; results do not apply to mortality on its own.[15]
Improving and maintaining risk status
The overall treatment goal in PAH is to achieve and maintain a low-risk status,[17] which has been shown to be prognostic for better long-term outcomes.[12][18][19] UPTRAVI® helps patients improve and maintain their risk status in both a clinical trial and the real world.[12][18][20]
In the GRIPHON trial, patients receiving UPTRAVI® were 69% more likely to improve their risk status from baseline to Week 26 compared with placebo.*‡[12]
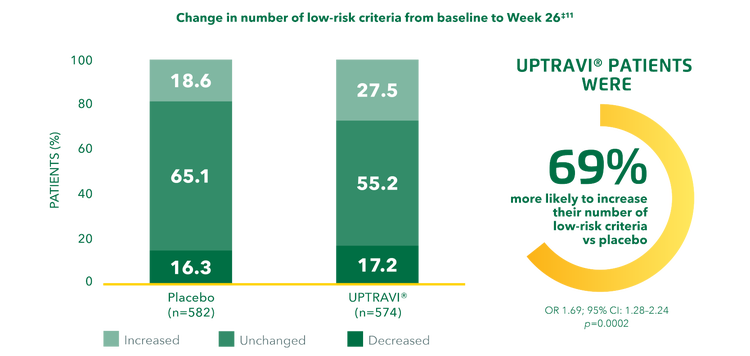
Adapted from Sitbon et al. 2020[12]
*Post hoc analysis assessing the effect of UPTRAVI® on risk profile from baseline to Week 26 compared with placebo in the GRIPHON population using both the non-invasive French registry and REVEAL 2.0 risk assessment methods.[12]
‡Change in number of low-risk criteria over time in the non-invasive French risk assessment subgroup.[12]
Regardless of risk group, the majority of patients with PAH improved or maintained their baseline risk status in the SPHERE registry.[21][20]
At 1 year:*‡[21]
- 22% of patients improved their risk status
- 63% of patients maintained their risk status
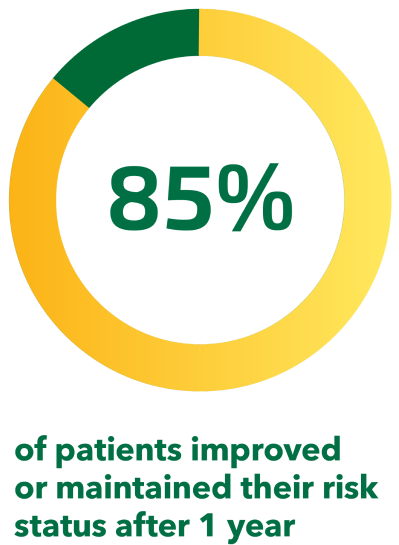
Adapted from Farber et al. 2019[21]
*From the first 250 patients enrolled in SPHERE, an ongoing US-based observational registry of UPTRAVI®-treated patients with PAH.[21]
‡Based on patients who had a risk assessment at both baseline and 1 year (196 patients or 78% of the total population).[21]
At 18 months:§#[20]
- 19% of patients improved their risk status
- 58% of patients maintained their risk status
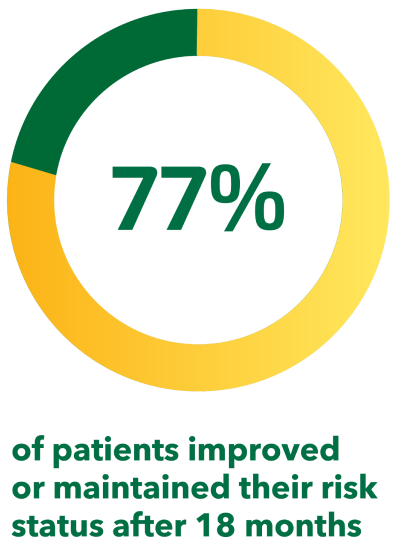
(This SPHERE analysis includes data from patients outside the therapeutic indications for UPTRAVI®; some markets may not be able to refer to this claim. If the market allows, the data may be switched to the poster by Farber H et al. Presented at ATS 2019 [Poster no. 10769])
Adapted from Kim et al. 2021[20]
§From the first 500 patients enrolled in SPHERE, an ongoing US-based observational registry of UPTRAVI®-treated patients with PAH.[20]
#Based on patients who had a risk assessment at both baseline and 18 months (388 patients or 78% of the total population). Data include a minority of patients outside the therapeutic indication for UPTRAVI®, including WHO FC I and WHO FC IV patients (n=51), patients from WHO PH groups 2–5 (n=26), as well as patients with PAH outside the indication (HIV [n=4], portal hypertension [n=18]).[20]
Continue reading
UPTRAVI® has a predictable and manageable safety profile.[1][3]
Adding UPTRAVI® is recommended for patients not fully at low risk with ERA + PDE-5i.[22][23]
Find resources such as PAH management guidelines and UPTRAVI® dosing and titration guides.
BMI, body mass index; CI, confidence interval; CHD, congenital heart disease; CTD, connective tissue disease; CV, cardiovascular; ERA, endothelin receptor antagonist; FC, functional class; HIV, human immunodeficiency virus; HPAH, heritable pulmonary arterial hypertension; HR, hazard ratio; IPAH, idiopathic pulmonary arterial hypertension; LVEDP, left ventricular end-diastolic pressure; OR, odds ratio; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PDE-5i, phosphodiesterase type 5 inhibitor; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; 6MWD, 6-minute walk distance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; WHO, World Health Organization; WU, Wood units
For further information regarding Opsumit® (macitentan) or Uptravi® (Selexipag) including full indications, all adverse effects and data please refer to the Israeli MOH prescribing information: https://israeldrugs.health.gov.il/#!/byDrug