Plan from the start to add UPTRAVI® within 6 months of diagnosis with pulmonary arterial hypertension (PAH), if the patient is not fully at low risk on dual therapy.[1][2][3][4]
On this page
When to initiate UPTRAVI®
According to the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines, the overall treatment goal in patients with pulmonary arterial hypertension (PAH) is to achieve and/or maintain a low-risk status,[1] which is associated with improved overall survival and functional capacity in patients.[5] Regular multiparameter risk assessment is recommended to evaluate patient risk status.[1]
2022 ESC/ERS guideline for sequential combination therapy with selexipag (UPTRAVI®)[1]

Adapted from Humbert et al. 2022[1]
Treatment with a prostacyclin receptor agonist allows for targeted treatment in PAH patients at risk of disease progression before initiation of parenteral therapy.[1][6] The 2022 ESC/ERS guidelines recommend that selexipag (UPTRAVI®) may be considered for patients on dual therapy who are at intermediate–high risk when the addition of IV/SC prostacyclin analogues is unfeasible.[1]

Expert consensus opinion on early UPTRAVI® initiation
Learn about the patient profiles identified as benefiting from UPTRAVI® initiation.[3]
Dosing and titration
The importance of individualised dosing
UPTRAVI® relaxes and widens the blood vessels by acting as an agonist at the prostacyclin (IP) receptors on the cellular surface. The density of these IP receptors is different amongst patients:[7]
- Patients with low IP receptor density require high IP receptor occupancy and are likely to require a higher dose of UPTRAVI®[7]
- Patients with high IP receptor density require low IP receptor occupancy and are likely to require a lower dose of UPTRAVI®[7]
Variations in IP receptor density from patient to patient can help explain the importance of an individualised maintenance dose for each patient.[7] Over the course of weeks, the dose of UPTRAVI® is gradually titrated, allowing the patient’s body to adjust to the new medication.[8]
Reaching the individualised maintenance dose
The recommended starting dose is 200 mcg twice daily. Tolerability may be improved when taken with food. Increase by 200 mcg twice daily, usually at weekly intervals, to the highest tolerated dose, up to 1600 mcg twice daily. If the dose is not tolerated, reduce to the previous tolerated dose.[8]
With individualised dosing, each patient can reach the dose that is best for them
If a dose of UPTRAVI® is missed, patients should take the missed dose as soon as possible unless the next dose is within the next 6 hours. If treatment is missed for 3 days or more, restart UPTRAVI® at a lower dose and then retitrate.[8]
The goal of treatment is to obtain optimal UPTRAVI® efficacy by titrating to the patient's individualised dose[9][10]

Patients achieved consistent outcome benefits across the range of UPTRAVI® doses in GRIPHON[11]
In the GRIPHON trial, UPTRAVI® delivered consistent efficacy across individualised maintenance dose groups, reducing the risk of disease progression vs placebo in patients with pulmonary arterial hypertension (PAH) receiving twice-daily (BID) low-, medium- and high-dose treatment.*[11]
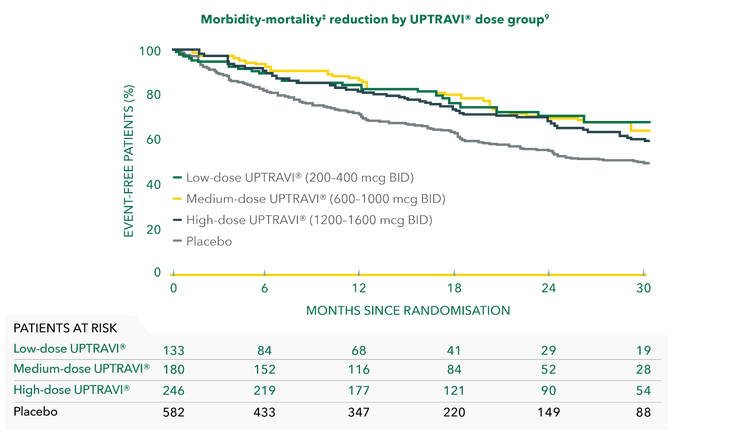
Adapted from Sitbon et al. 2015[11]
*Low-dose group (HR 0.60; 95% CI: 0.41–0.88), medium-dose group (HR 0.53; 95% CI: 0.38–0.72), high-dose group (HR 0.64; 95% CI: 0.49–0.82).[11]
‡Composite primary endpoint; results do not apply to mortality on its own.[6]
Managing side effects during the titration period
The adverse effects of UPTRAVI® are a marker of IP receptor stimulation and, therefore, of drug activity.[12] Tolerability of these adverse effects helps to determine each patient’s individualised maintenance dose of UPTRAVI®.[6][8][13]
Managing UPTRAVI® adverse effects can be straightforward with over-the-counter medications, prescription medications and/or non-pharmacological interventions.[8][13][14]
Continue reading
UPTRAVI® has a predictable and manageable safety profile.[9][12]
Learn about the patient profiles identified as benefiting from UPTRAVI® initiation.[3]
Guidance on correctly starting your patient on their UPTRAVI® journey.
BID, twice daily; CI, confidence interval; DPAH, drug- or toxin-associated pulmonary arterial hypertension; ERA, endothelin receptor antagonist; ERS, European Respiratory Society; ESC, European Society of Cardiology; HPAH, heritable pulmonary arterial hypertension; HR, hazard ratio; IP, prostacyclin; IPAH, idiopathic pulmonary arterial hypertension; IV, intravenous; PAH, pulmonary arterial hypertension; PDE-5i, phosphodiesterase type 5 inhibitor; SC, subcutaneous
For further information regarding Opsumit® (macitentan) or Uptravi® (Selexipag) including full indications, all adverse effects and data please refer to the Israeli MOH prescribing information: https://israeldrugs.health.gov.il/#!/byDrug