Stelara® Delivers Rapid Efficacy In UC
In UC, STELARA® Delivers... Efficacy From A Single Iv Dose
1 Dose of STELARA® reduced the symptoms† of UC as early as Week 22
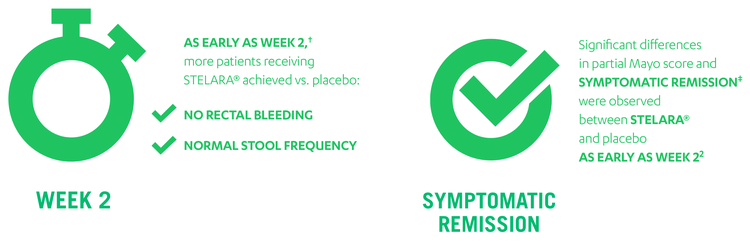
† Week 2, the earliest scheduled study visit, and at each visit thereafter during UNIFI induction study.
‡ Symptomatic remission is defined as a Mayo stool frequency sub-score of 0 or 1 and a rectal bleeding sub-score of 0.
~87% Of Biologic Non-Failure Patients Achieved Clinical Response With STELARA® At Either Week 8 Or 16 [1]
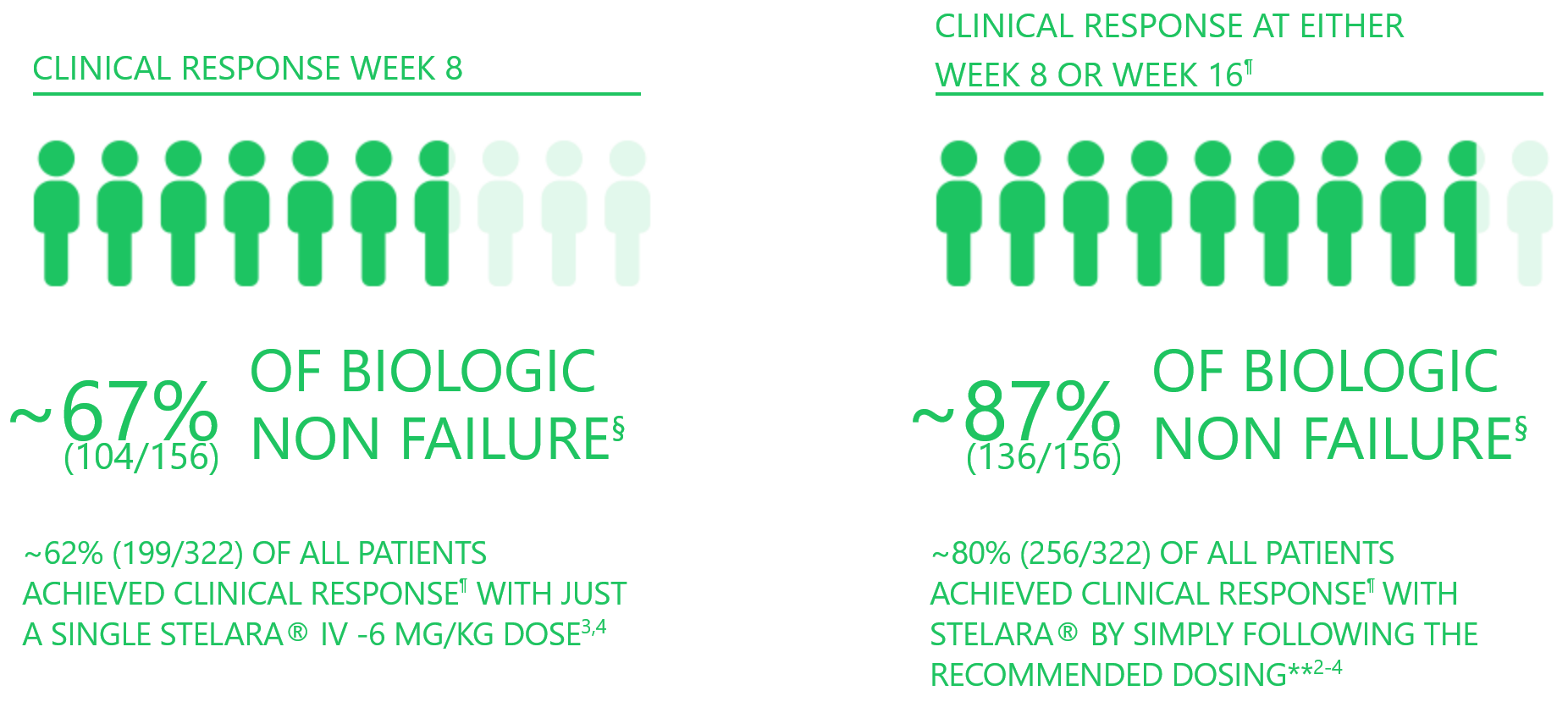
§ 93.4% biologic naive.
¶ Decrease from induction baseline in Mayo score (stool frequency, rectal bleeding, finding on flexible proctosigmoidoscopy and physician's global assessment) by >30% and >3 points, with either a decrease from induction baseline in rectal bleeding sub-score of 0 or 1.
** Clinical response at either Week 8 or Week 16. Patients not in clinical response at Week 8 received STELARA® 90 mg SC at Week 8 (as per recommended product dosing) and were re-analysed for clinical response at Week 16. Patients were counted only once if they achieved response at Weeks 8 and 16.
Component not published? componentType is undefined.
Component not published? componentType is undefined.
* Long-term remission is considered a target of ideal treatment in the clinical management of ulcerative colitis (UC).[2]
IV: Intravenous; UC: Ulcerative colitis; SC: Subcutaneous.
Prescribing Information
Adverse Events Reporting